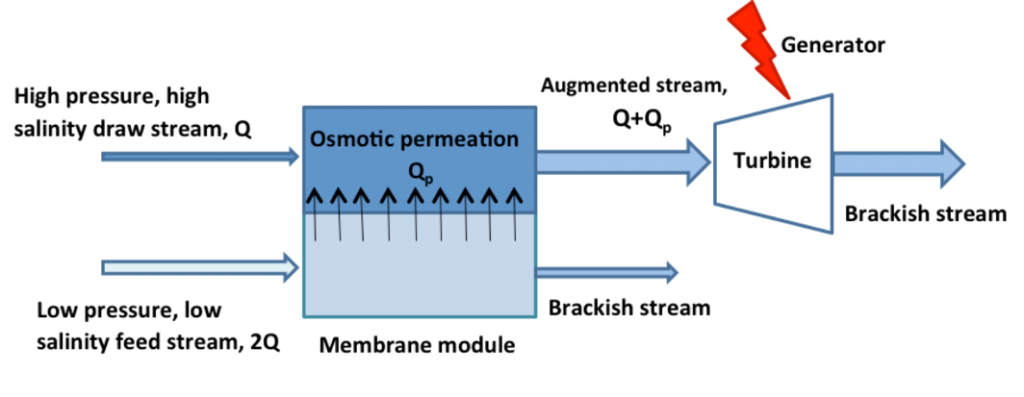
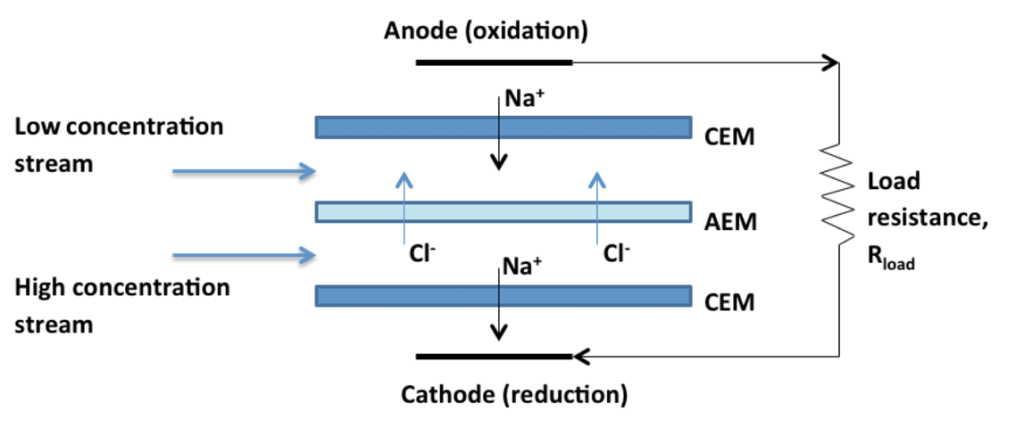
When two streams of different concentration are mixed, energy is released. Membranes may be used to tap this clean and renewable source of energy by controlling the transport of species across a semi-permeable, selective barrier. In the case of salinity gradients, for example seawater and freshwater, one may transport either water – via osmosis – or ions.
The first case, where water is transported across an osmotic membrane, is analogous to energy production by a hydro-turbine. As water passes from a dilute stream into a concentrated, pressurized stream, the now volume-augmented stream is used to turn a turbine blade and generate electricity (see schematic diagram below). This process is known as `Pressure-retarded Osmosis’, or PRO, and is the exact inverse of Reverse Osmosis, used to separate salt from water…
In the second case, ion-selective membranes allow transport of anions or cations across a ‘cell’ consisting of alternating membranes and outer electrodes (see schematic below). The ion flux is converted into an electron flux (Faradaic reaction) at the electrode and, when connected to an external circuit, produces electrical power. The diffusion-driven process is known as Reverse Electrodialysis (RED).
Publications:
- Scale-up characteristics of membrane-based salinity-gradient power production
Feinberg, B.J., Ramon, G.Z., Hoek, E.M.V., Journal of Membrane Science, 2015 - A thermodynamic analysis of osmotic energy recovery at a Reverse Osmosis desalination plant
Feinberg, B.J., Ramon, G.Z., Hoek, E.M.V., Environmenatal Science & Technology, 2013 - Membrane-based production of salinity-gradient power
Ramon, G.Z., Feinberg, B.J., Hoek, E.M.V., Energy & Environmental Science, 2011